[Drivetrain] [Brakes] [Suspension] [Steering] [Electrical] [Emissions]
QUESTIONS – Emissions |
||||
We are going to look at:
As always – if something seems unclear, it probably is – so ask me for clarification (then I can make it more clear for others) |
||||
HISTORY |
||||
First, always remember: you need to breathe this air.
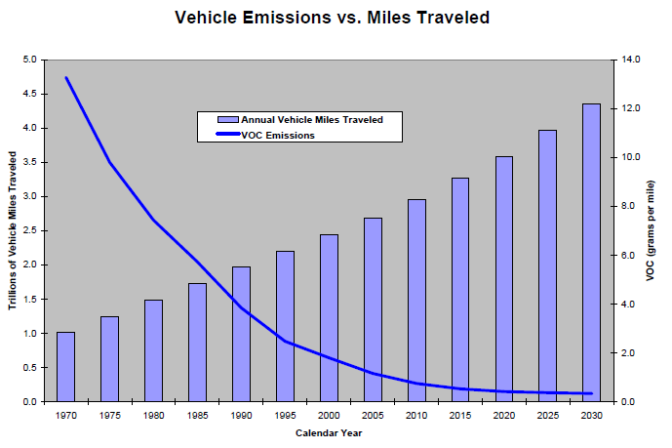
The exhaust emitting from the tailpipe of a vehicle isn’t something you want to be breathing in. All vehicles pollute, and the automobile is blamed for a large part of Earth’s environmental damage. Thankfully, due to improvements in controlling those emissions, the level of pollutants have been greatly reduced.
The name “Smog” was first used in 1905, by Dr. H.A. Des Voeux of London’s Coal Smoke Abatement Society: a combination of “smoke” and “fog,” used to describe the air quality in coal-burning London. While we generally do not drive coal-powered vehicles, the term is still used today. Tetraethyl lead was used as a gasoline additive in 1923 as it was a very good valve seat lubricant and a good octane booster. Lead is very hazardous to human health, but this did not stop automobile manufacturers from encouraging the use of leaded fuels. Lead does not break down once it is in the environment or in your body. Environmental historian Ted Steinberg noted, “With the burning of huge quantities of gasoline (especially in the three decades after 1950), lead was deposited on the soil and, unknowingly, tracked into houses across the nation. Infants crawling on the floor then picked it up on their fingers and ingested it, interfering with the development of their nervous systems and contributing to hyperactivity and hearing loss, among other effects, although it would be decades . . . before the full scope of the problem became evident.” From the 1920s until the late 1980s, seven million tons of lead was spewed out by cars across the country. I grew up around leaded gas. I also worked at a full-serve gas station right out of highschool, and could identify leaded, unleaded, and premium, just by the smell alone. The fuel filler inlets were made smaller on unleaded cars than the leaded cars, preventing you being able to fill up with the leaded nozzle. Except leaded gas was cheaper, so many people punched out the filler opening to fit the larger leaded fuel nozzle. Leaded gas quickly polluted the catalytic converters (we’ll talk “cats” later).
In the 1940s, people in Los Angeles complained about a white or yellow-brown haze that made their eyes tear (crying, not ripping).
In 1948 the air pollution from a Steel Mill in Donora Pennsylvania, held close to the ground during a temperature inversion (smog), made 43% of the townsfolk ill, 10% severely affected and 19 people died. This incident opened the eyes of the public to the importance of air quality. This was later repeated in 1952 in London England, when 4000 people died because of “Killer Smog,” and again in 1953 in New York when 200 died as a result of smog. It wasn’t until 1955 that research began on air pollution, and until 1965 that the automobile’s pollution had environmental standards to meet. For the next 12 years or so, automobile manufacturers and the Environmental Protection Agency struggled to reach a happy medium, made worse by the 1973 Energy Crisis requiring more fuel efficient automobiles. Today, the environmental impact of the automobile has contributed to headaches, lung cancer, emphysema, various other respiratory and cardiovascular problems, and have been linked to low birth weight in infants. They also modify weather conditions, damage vegetation, and eat away at rubber, textiles, dyes, and other materials. It is for these reasons, that we must do our best to control the emissions from our vehicles.
|
||||
CHEMICALS & POLLUTANTS |
||||
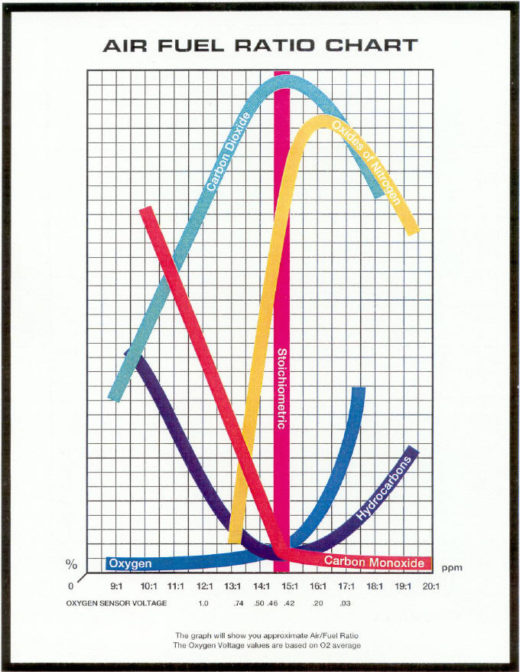
| In a perfect world, the Hydrocarbons (HC) in the fuel combine with the oxygen (O2) in the air to produce Water (H2O) and Carbon Dioxide (CO2).
HC + O2 = H2O + CO2
Unfortunately, this world is not perfect: Fuel is not strictly HC, and air is not strictly O2.
All these chemicals combine during combustion to produce the following nasty chemicals: Carbon Dioxide (CO2): Should be high – it is a by-product of perfect combustion. Decreases the further we are from stoichiometric. CO2 is a by-product of perfect combustion. Yes, it’s a “greenhouse gas,” but trees love it! Oxygen (O2): Should be low – all the O2 we give it should have combined with HC. Increases as engine runs LEAN (if the engine is not getting enough fuel, unused oxygen is left over). O2 is more of a tuning aid than a pollutant – humans love it!. Carbon Monoxide (CO): Should be low. CO is a result of incomplete combustion: there is so much HC that it forgot to keep burning. Mechanical faults as well as tuning can be blamed. Increases as engine runs rich, because not all the fuel is burning completely Hydro Carbons (HC): Should be low – perfect combustion should burn all of it. HC is a result of unburned fuel. It increases the further we are from stoichiometric because not all the fuel is being burned. Can be reduced with higher combustion temperatures. Oxides of Nitrogen (NOx): Should be low. Caused by high combustion temperatures. Largest contributor to smog. Attempts to reduce NOx usually result in increased HC, and vice-versa.
MrWELLWOOD’S LESSON ON EMISSION CONTROLS:
|
||||
CONTROLS |
||||
|
When automakers made engines back in the day, they were built to perform. Gas was cheap; we weren’t concerned about air quality or global warming; and big engines were only bested by bigger engines. The Muscle Car era came to an end with the oil shortage of 1973 and the increased emission requirements of the 1970’s. These engines could not even touch the power and efficiency that today’s engines can. There are two ways to deal with engine emissions: Design the engine for efficiency Add systems to manage pollutants Domestic manufacturers chose to add systems to the vehicle, while imports improved the engine design to deal with pollution at the source – the engine. With today’s modern computer-controlled engines, HUGE GAINS in fuel economy AND power AND emission controls are possible! YOU are right now actually living in the MOST TECHNOLOGICALLY ADVANCED day of the automobile! NEVER have vehicles made as much power, had such good fuel economy, and still saved the environment like today.
|
||||
POSITIVE CRANKCASE VENTILATION (PCV) |
||||
1963
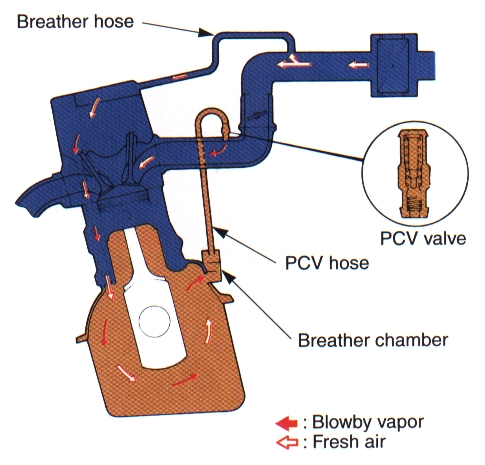
In Level 1 Engine Blocks you learned about piston rings, and you measured the piston ring gaps in your engines. There MUST be a gap of you will break pistons when the ring expand from heat, but that also means there will be compression, combustion, and oil leaks through those gaps. Combustion getting past the rings is called “blow by” and is basically exhaust getting into your crankcase – this is NOT good for your oil or bearings. It was discovered in 1952 that these vapours were also a primary component of smog. That’s not good for the environment! (Or your engine oil!!!). My 1961 Chevy pickup originally came with a “road draft tube:” just a pipe that dumped all the blow-by out onto the ground. The “environment” hadn’t been invented yet.
What we did was actually draw those fumes back into the engine and burn them:
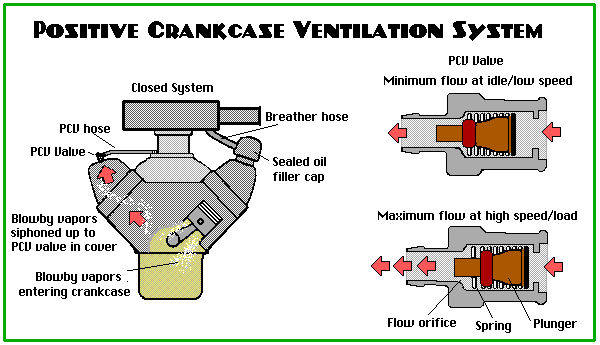
WITHOUT PCV – Crankcase vapours (“blow-by” – the stuff that “blows by” the rings) react with the oil and form sludge, which corrodes and accelerates the wear of the internal engine parts. WITH PCV – The system draws fresh filtered air into the crankcase, collects the nasty vapours, fumes, unburned gasoline, moisture, and yuck from the crankcase, and draws them into the intake manifold where they are burned back in the engine. This “flow” of air through the crankcase actually works well for us and the life of our engine! The PCV System uses a one-way check valve (called a PCV Valve) that is partially closed at idle and low speed to allow some air flow through the crankcase. It opens more at higher speeds to draw more contaminants out of the system. At full throttle there is no vacuum in the system, so the extra blow by gasses go back up through the fresh air inlet and get drawn into the incoming intake air stream. The valve closes under backfire so we don’t ignite anything inside the crankcase. You can tell if your engine has junk rings by looking at where the fresh air filter is on the engine – if it’s dripping with oil, that means weak rings are pushing tons of oil back up to the filter. The drift-boy-wannabe’s will tell you to install an oil separator to prevent that – I will tell you to fix the rings..
CAUTION: Deleting PCV system can result in excessive crankcase pressure, causing oil leaks, oil consumption, poor driveability, and engine longevity problems (Oil Sludge will collect inside the motor; sludge does NOT lubricate well). Contributes to better fuel economy Contributes to longer engine life Minimal effect at full throttle (excessive blow-by gets pushed into the airstream) Reduction of HC
IS YOUR ENGINE JUNK? Worn piston rings will allow combustion gasses to blow by the rings (called “Blow-By”). If the rings are junk, they will push extra “blow by” up through the breather hose and into the incoming air charge. This blow-by is going through the crankcase, so it will pick up oil vapour, and deposit an oily residue around the filter on the breather hose. An oil-soaked PCV filter is an indicator of worn engine Incidentally, a faulty PCV System can CAUSE an engine to burn oil – make sure the system is working correctly before you rush out to rebuild that motor. The Drift-Boy-Wannabe’s will tell you to install an oil separator, but –I- will tell you to fix the rings.
I deleted this system in my Lotus Super 7 replica when I converted it to ITBs. The engine oil gets dark SUPER quicky, and I have to change it frequently (like once a month!!). I’m going to put it back when I put the bigger engine in.
|
||||
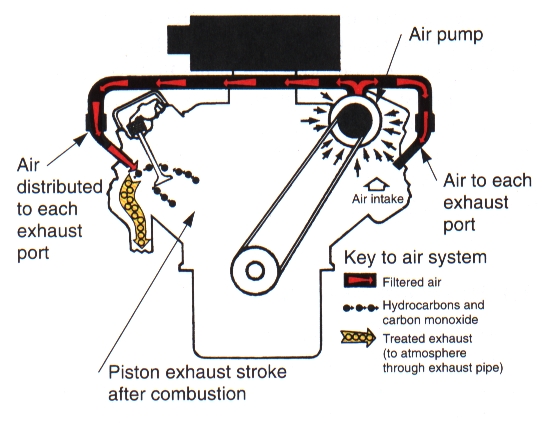
AIR INJECTION REACTOR (AIR) |
||||
1968Our engines are not totally efficient, especially engines back in the old days. If an engine is not efficient, some of the fuel may leave the combustion chamber only partially burnt. This system works kind of like blowing on a campfire to get it going – It blows air into the exhaust system to help complete the combustion of the fuel.
Since no engine is 100% efficient, there is always some unburned fuel in the exhaust itself. Combustion requires fuel, heat and oxygen. Since exhaust is hot, and there is some unburned fuel in it, if we add oxygen to the mix, we can continue burning the exhaust. Air is pumped into the exhaust right at the port which ignites any leftover fuel. This combustion does not make any power for us, but you may hear faint “popping” from the exhaust, moreso if the mixture is very rich. The AIR system has been largely discontinued since the 1990’s. Small parasitic draw of the AIR pump (ie: Costs you horsepower always) Reduction of HC and CO
|
||||
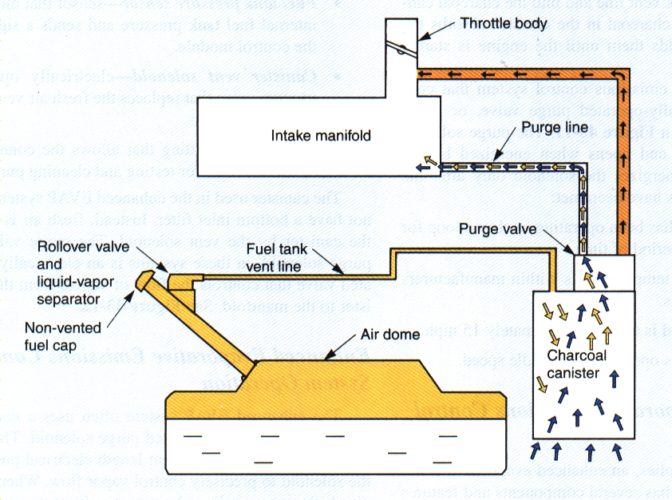
EVAPORATIVE EMISSIONS |
||||
1971In Level 1 Fuel Systems, you learned that we need heat for fuel to vapourize. You probably already know that if you spill gas on the ground in summer, it evapourates (vapourizes) really quickly. The fuel in your gas tank will evapourate too, even while you’re parked! The gas tank must have a vent to let air in when you’re pumping fuel out (or you will eventually create more vacuum in the tank than the pump can provide), and our precious dinosaur elixir will evapourate through that vent. Turns out that fuel vapour isn’t good for the environment. This system takes the fuel vapours from the gas tank that would have floated off into the atmosphere, and stores them in a charcoal canister to be burned by the engine during cruise. This system is like “free fuel economy” – you have nothing to lose and everything to gain by keeping it. The tank is still vented – it just vents through charcoal Modern cars use one or more solenoids to control the flow of these fumes. These solenoids can be problematic as they fail. Sometimes they pose no driveability problem, but sometimes they make the car not want to start after fueling (the engine is flooded with fumes!).
Does not operate under full throttle Contributes to better fuel economy Reduction of HC
|
||||
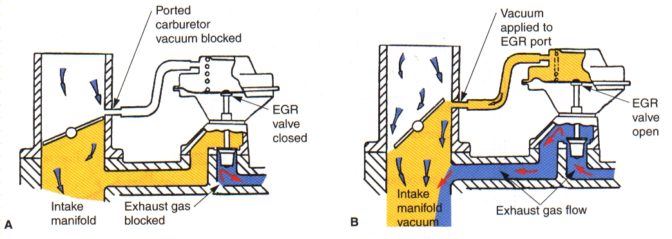
EXHAUST GAS RECIRCULATION (EGR) |
||||
1973Fuel needs heat to burn. That’s good: Running a hotter engine, and running higher combustion temperatures, can reduce HC emissions – also good! However, it’s also a problem: Oxides of Nitrogen (NOx) is a result of high combustion temperatures. NOx reacts with sunlight to create photochemical smog. This is the brown haze you see. In the 1970’s manufacturers (notably Ford) altered camshaft timing to reduce cylinder pressures in an effort to reduce NOx. This also reduced power throughout the engine’s operating range (the 1975 Mustang’s V8 made 145hp). Since, truthfully we spend the most of our driving cruising, if we can reduce combustion temperatures at cruise, we can reduce NOx. We can reduce combustion temperatures by filling part of the cylinder with an inert “fill” that does nothing (inert means “non-reactive” – basically means “does nothing”; it does not add to combustion, it does not detract from combustion).
The EGR valve takes takes some of the exhaust gasses from the exhaust (inert and non-combustible) and runs them back into the engine to dilute the mixture and reduce temperatures. This is kind of like packing peanuts in a cardboard box – it isn’t what you ordered, it does nothing to the product, but it fills up the container. Feeding exhaust gasses through the engine does not contribute to power, so it cannot operate all the time. It must be metered through an EGR valve. The EGR valve responds to high-vacuum/low-throttle-position (cruise), keeping it closed for cold start, idle and full throttle. Any unburned hydrocarbons in the exhaust could also be burned in the engine. At full throttle (zero manifold vacuum), the system shuts off giving you full cylinder filling and full-power. Since the incoming air charge has a portion of exhaust mixed with it, the fuel molecules are farther apart. This means it will take longer for all of them to burn, so we will ADD some more spark advance (Level 1: Ignition System) to make sure we have enough time to get everything burned. Back in the day Honda was able to meet emission standards on their CVCC engine without an EGR system or a Catalytic Converter – just good engine design! Today many manufacturers are also able to meet emission standards without the use of EGR – by better designed combustion chambers! CAUTION: Deleting the EGR system AND NOT altering the tune, can result in DETONATION, since the spark is now happing WAY too early – this can destroy your engine! Does not operate under full throttle Contributes to better fuel economy Reduction of NOx
You’re going to find a lot of interweb hate on the EGR, and all “cool kids” delete this. They will say that the EGR makes their intake all yucky. Truthfully, the intake is yucky because their engine is burning oil and oily exhaust is getting into the motor. Keep the system in there – it does not hurt you. And just fix the oil burning issue!
|
||||
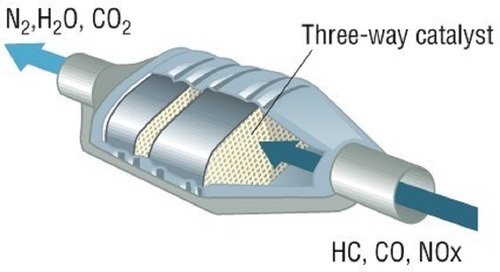
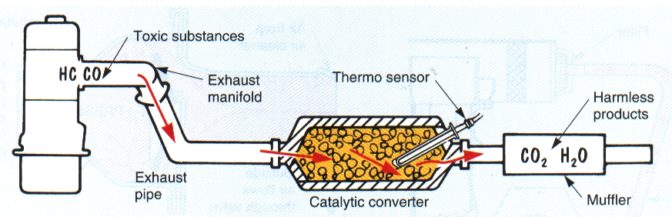
CATALYTIC CONVERTOR |
||||
1975Uses rare Earth metals to further burn pollutants in the exhaust with a chemical reaction. This is the one emissions device I do not pass around the class for kids to look at and touch – kinda toxic.
It looks like a muffler in the exhaust system quite close to the engine. It uses unique rare metals to cause a chemical reaction to burn anything that didn’t get burned in the engine. Pellets or a honeycomb made of platinum or palladium react (in the presence of oxygen) with the hydrocarbons and carbon monoxide converting them to carbon dioxide and water. An egg or sulfur smell is not uncommon – this indicates either an out-of-tune engine, or a high sulfur content fuel. Modern cats also use Rhodium as a reducer instead of a catalyst (reactor) to reduce Oxides of Nitrogen by removing the oxygen component, thus releasing Nitrogen. These rare Earth metals bring a LOT of money, so Catalytic Converts get stolen! Also – almost all the cats in the junkyard get bought for cheap, and then sold to the recycler for way WAY more – lucrative!
The chemical reaction generates heat. The dirtier the exhaust: the harder the converter works: the hotter it gets. Some badly out-of-tune cars have set fire to either the ground or themselves by the heat of the converter. In the bad old days, the early cats were heavy and did not flow well. The would plug up real fast if you were burning leaded gas (which is why we have unleaded gas now), and they choked your power. Today, cats flow really well, and removing them does not significantly increase your power. I’ve run “High Flow” cats to get performance and stay legal and eco-friendly, they are not that expensive (and not as “good” or as “long lasting” as factory cats). It is illegal to delete a catalytic converter. “Federal Offense” illegal. OBD-II systems use a second O2 sensor after the Cat to check if the cat is working correctly. A Malfunction Indicator Lamp (MIL) will light if it is not working (or missing). Honda was able to meet emission standards on their CVCC engine back in the day without an EGR system or a Catalytic Converter – just good engine design! Negligible exhaust restriction (if in good shape) Reduction of HC and CO (and now NOx with a 3-way Cat)
|